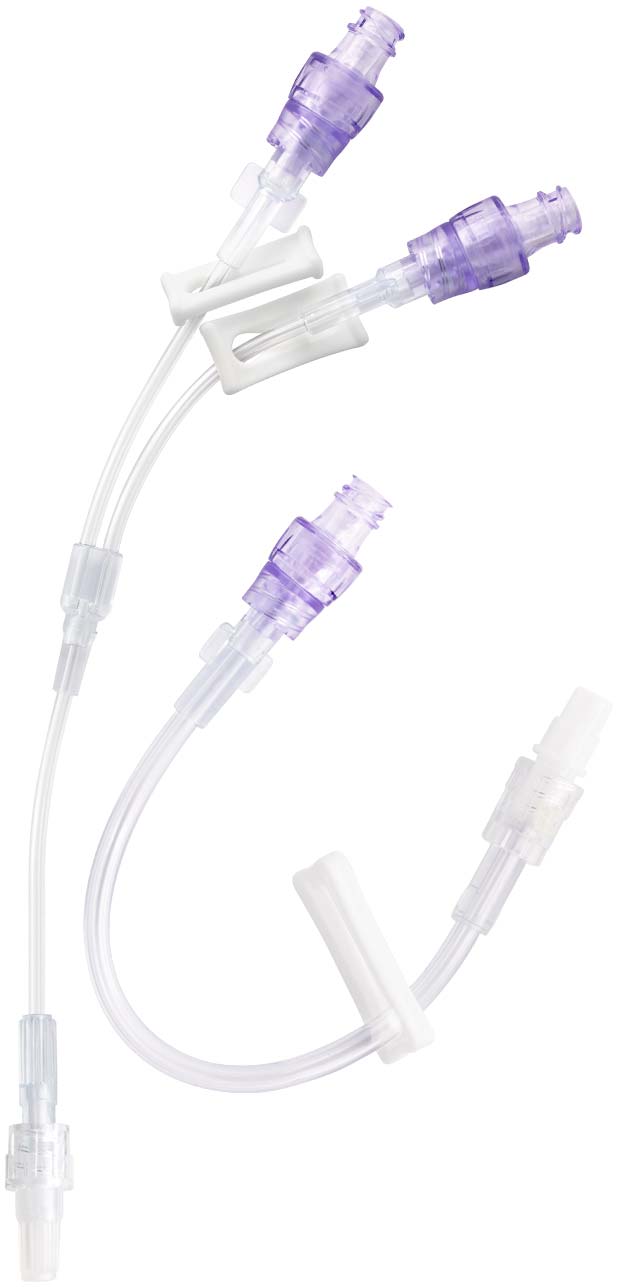
Multiple Configurations for Clinical Use
Manufactured with the highest quality, nPulse Extension Sets with neutral needle-free connectors are available in several configurations. We offer various lengths and tubing diameters (microbore and macrobore) to meet diverse applications in a range of clinical settings. The nPulse Needle-Free Connector features neutral fluid displacement at both luer connection and disconnection.